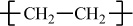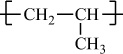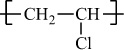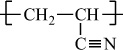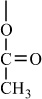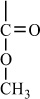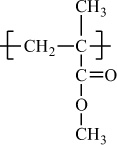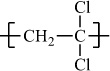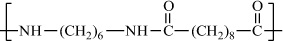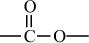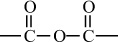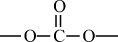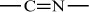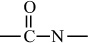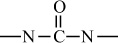Introduction to Polymer Science
- 1.1 Classification of Polymers
- 1.2 Polymer Structure
- 1.3 Molecular Weight
- 1.4 Chemical Structure and Thermal Transitions
- Suggested Reading
- Problems
The word polymer is derived from the classical Greek words poly meaning “many” and meres meaning “parts.” Simply stated, a polymer is a long-chain molecule that is composed of a large number of repeating units of identical structure. Certain polymers, such as proteins, cellulose, and silk, are found in nature, while many others, including polystyrene, polyethylene, and nylon, are produced only by synthetic routes. In some cases, naturally occurring polymers can also be produced synthetically. An important example is natural (Hevea) rubber, known as polyisoprene in its synthetic form.
Polymers that are capable of high extension under ambient conditions find important applications as elastomers. In addition to natural rubber, there are several important synthetic elastomers including nitrile and butyl rubber. Other polymers may have characteristics that enable their fabrication into long fibers suitable for textile applications. The synthetic fibers, principally nylon and polyester, are good substitutes for naturally occurring fibers such as cotton, wool, and silk.
In contrast to the usage of the word polymer, those commercial materials other than elastomers and fibers that are derived from synthetic polymers are called plastics. A typical commercial plastic resin may contain two or more polymers in addition to various additives and fillers. These are added to improve a particular property such as processability, thermal or environmental stability, or mechanical properties.
The birth of polymer science may be traced back to the mid-nineteenth century. In the 1830s, Charles Goodyear developed the vulcanization process that transformed the sticky latex of natural rubber into a useful elastomer for tire use. In 1847, Christian F. Schönbein reacted cellulose with nitric acid to produce cellulose nitrate. This was used in the 1860s as the first man-made thermoplastic, celluloid. In 1907, Leo Hendrik Baekeland 1 produced Bakelite (phenol−formaldehyde resin). Glyptal (unsaturated-polyester resin) was developed as a protective coating resin by General Electric in 1912.
By the 1930s, researchers at DuPont in the United States had produced a variety of new polymers including synthetic rubber and more “exotic” materials such as nylon and Teflon. By 1938, Dow had produced polystyrene in commercial scale for the first time and, in 1939, polyethylene (low-density) was made by scientists at ICI in England. Efforts to develop new polymeric materials, particularly synthetic rubber, were intensified during World War II when many naturally occurring materials such as Hevea rubber were in short supply. In the 1950s, Karl Ziegler and Giulio Natta independently developed a family of stereospecific transition-metal catalysts that made possible the commercialization of polypropylene as a major commodity plastic. The 1960s and 1970s witnessed the development of a number of high-performance engineering plastics polymers that could compete favorably with more traditional materials, such as metals, for automotive and aerospace applications. These included polycarbonate, poly(phenylene oxide), polysulfones, polyimides, aromatic polyamides such as Kevlar, and other high-temperature rigid-chain polymers. More recently, specialty polymers with electrically conducting, photoconducting, and liquid-crystalline properties have appeared for a variety of applications.
Today, polymeric materials are used in nearly all areas of daily life and their production and fabrication are major worldwide industries. The annual U.S. production of plastics and synthetic fibers in 2012 and the average annual change in production over the decade from 2002 to 2012 are given in Table 1-1. In 2012, the total U.S. production of synthetic fibers (principally non-cellulosic) and plastics was 2.81 and 34.1 million metric tons, respectively. Among plastics, the largest shares of the total production in 2012 were the polyethylenes, followed by polypropylene, poly(vinyl chloride) (PVC), and polystyrene. Although not specifically listed by the data given in Table 1-1, thermosetting resins (principally phenolic, urea, and melamine resins) typically represent around 10% of the total plastics production while synthetic rubbers, such as styrene–butadiene rubber (SBR) and polybutadiene, represent only about 6% of the total production in recent years.
Table 1-1 U.S. Production of Major Plastics and Synthetic Fibers in 2012a
Thousands of Metric Tonsb |
Annual Change (%), 2002-12 |
||
PLASTICS |
|||
Polyethylene |
|||
|
3123 |
-1.5 |
|
|
8098 |
1.7 |
|
|
8046 |
1.1 |
|
Polypropylene |
7405 |
-0.4 |
|
Polystyrene |
2473 |
-2.0 |
|
PVC & copolymers |
6944 |
0.0 |
|
SYNTHETIC FIBERS |
|||
Non-cellulosic |
|||
|
562 |
-6.6 |
|
|
1021 |
-3.1 |
|
|
1203 |
-2.2 |
|
Cellulosic |
|||
|
27 |
-10.3 |
|
1.1 Classification of Polymers
Thousands of different polymers have been synthesized and more will be produced in the future. Conveniently, all polymers can be assigned to one of two groups based upon their processing characteristics or the type of polymerization mechanism. A more specific classification can be made on the basis of polymer structure. Such groupings are useful because they facilitate the discussion of properties.
1.1.1 Thermoplastics and Thermosets
All polymers can be divided into two major groups based on their thermal processing behavior. Those polymers that can be heat-softened in order to process into a desired form are called thermoplastics. Waste thermoplastics can be recovered and refabricated by application of heat and pressure. Polystyrene is an important example of a commercial thermoplastic. Other major examples are the polyolefins (e.g., polyethylene and polypropylene) and poly(vinyl chloride). In comparison, thermosets are polymers whose individual chains have been chemically linked by covalent bonds during polymerization or by subsequent chemical or thermal treatment during fabrication. Once formed, these crosslinked networks resist heat softening, mechanical deformation, and solvent attack, but cannot be thermally processed. Such properties make thermosets suitable materials for composites, coatings, and adhesive applications. Principal examples of thermosets include epoxy, phenol–formaldehyde resins, and unsaturated polyesters that are used in the manufacture of glass-reinforced composites such as Fiberglas (see Section 7.4).
1.1.2 Classification Based upon the Mechanism of Polymerization
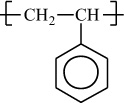
In addition to classifying polymers on the basis of their processing characteristics, polymers may also be classified according to their mechanism of polymerization. An early scheme classifies polymers as either addition or condensation—a scheme attributed to Wallace Carothers 2, a pioneer of the polymer industry working at DuPont from 1928 until his untimely death in 1937. Polystyrene, which is polymerized by a sequential addition of styrene monomers (see Figure 1-1), is an example of an addition polymer. Most important addition polymers are polymerized from olefins and vinyl-based monomers. A few other polymers that are traditionally recognized as belonging to the addition class are polymerized not by addition to an ethylene double bond but through a ring-opening polymerization of a sterically strained cyclic monomer. An example is the ring-opening polymerization of trioxane to form polyoxymethylene (an engineering thermoplastic), which is illustrated in Figure 1-2. Table 1-2 lists the chemical structure of the repeating units and the commonly used nomenclature of some of the most important addition-type polymers derived from substituted ethylene.
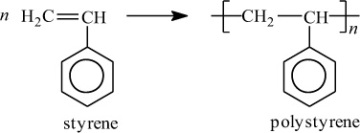
Figure 1-1 Polymerization of styrene.
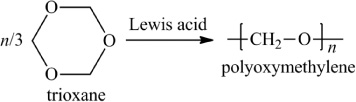
Figure 1-2 Ring-opening polymerization of trioxane.
Table 1-2 Examples of Some Important Addition Polymers Derived from Ethylene
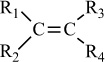
Polymer |
R1 |
R2 |
R3 |
R4 |
Repeating Unit |
Polyethylene |
H |
H |
H |
H |
|
Polypropylene |
H |
H |
H |
CH3 |
|
Poly(vinyl chloride) |
H |
H |
H |
Cl |
|
Polyacrylonitrile |
H |
H |
H |
C≡N |
|
Poly(vinyl acetate) |
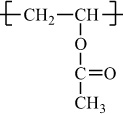
H |
H |
H |
|
|
Polystyrene |
H |
H |
H |
|
|
Poly(methyl methacrylate) |
H |
H |
CH3 |
|
|
Poly(vinylidene chloride) |
H |
H |
Cl |
Cl |
|
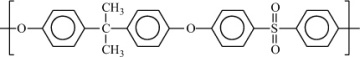
Condensation polymers are obtained by the random reaction of two molecules. A molecule participating in a polycondensation reaction may be a monomer, oligomer, or higher-molecular-weight intermediate each having complementary functional end units, such as carboxylic acid or hydroxyl groups. Typically, condensation polymerizations occur by the liberation of a small molecule in the form of a gas, water, or salt. Any high-yield condensation reaction such as esterification or amidation can be used to obtain a high-molecular-weight polymer. An example of a condensation polymerization is the synthesis of nylon-6,6 by the polycondensation of adipic acid and hexamethylenediamine as illustrated in Figure 1-3A. This polymerization is accompanied by the liberation of two molecules of water for each repeating unit. Another important example of a polycondensation, illustrated in Figure 1-3B, is the preparation of polycarbonate from bisphenol-A and phosgene. In this case, two molecules of hydrogen chloride are formed for each repeating unit. Alternatively, if the sodium salt of bisphenol-A was used in the polymerization, the by-product of the condensation would be sodium chloride rather than hydrogen chloride. The salt will precipitate out of the organic solvent used for the polymerization and, therefore, can be easily and safely removed. Some other examples of condensation polymers are given in Table 1-3.
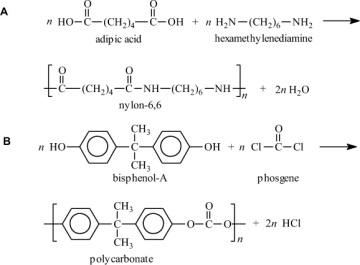
Figure 1-3 Two examples of a condensation polymerization. A. Polyamidation of nylon-6,6. B. Polymerization of bisphenol-A polycarbonate.
Table 1-3 Examples of Some Condensation Thermoplastics
Polymer |
Repeating Unit |
Polysulfone |
|
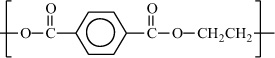
Poly(ethylene terephthalate) |
|
Poly(hexamethylene sebacamide) (nylon-6,10) |
|
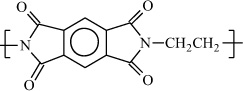
Poly(ethylene pyromellitimide) |
|
More recently, another classification scheme based on polymerization kinetics has been adopted over the more traditional addition and condensation categories. According to this scheme, all polymerization mechanisms are classified as either step growth or chain growth. Most condensation polymers are step growth, while most addition polymers are chain growth; however, a number of important exceptions exist, as will be discussed in Chapter 2. During chain-growth polymerization, high-molecular-weight polymer is formed early during the polymerization, and the polymerization yield, or the percent of monomer converted to polymer, gradually increases with time. In step-growth polymerization, high-molecular-weight polymer is formed only near the end of the polymerization (i.e., at high monomer conversion, typically >98%). Details of the mechanisms for chain-growth and step-growth polymerizations are discussed in Chapter 2.
1.1.3 Classification Based upon Polymer Structure
In addition to classification based upon processing and polymerization characteristics, polymers may also be grouped based upon the chemical structure of their backbones. For example, polymers having all carbon atoms along their backbone are important examples of homochain polymers. They may be further classified depending upon whether there are single or double bonds along their backbone. Carbon-chain polymers with only single bonds along the backbone are called polyalkylenes (or polyalkylidenes). Examples of polyalkylenes include polystyrene, the polyolefins (e.g., polyethylene and polypropylene), and poly(vinyl chloride). Carbon-chain polymers with double bonds along the chain such as the diene elastomers—polyisoprene and polybutadiene—are called polyalkenylenes. Another example of a polyalkenylene is polyacetylene, an electrically conducting polymer (see Section 10.2.7).
Heterochain polymers that contain more than one atom type in their backbone are grouped according to the types of atoms and chemical groups (e.g., carbonyl, amide, or ester) located along the backbone. The most important classes of organic heterochain polymers are listed in Table 1-4. Another important class of heterochain polymers includes polysiloxanes. These have a –Si–O– backbone with methyl or other substituent groups attached to silicon.
Table 1-4 Backbone Structures of Some Important Organic Heterochain Polymers
Polymer Classification |
Backbone Group |
Carbon–Oxygen Polymers |
|
|
|
|
|
|
|
|
|
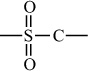
Carbon–Sulfur Polymers |
|
|
|
|
|
Carbon–Nitrogen Polymers |
|
|
|
|
|
|
|
|
|