Problems
A. Discussion Problems
A1. The Fenske equation:
Is valid only for binary systems.
Was derived for minimum reflux.
Requires CMO.
Requires constant K values.
All of the above.
None of the above.
A2. If you want to use an average relative volatility, how do you calculate it for the Underwood equation?
A3. Develop your key relations chart for this chapter.
A4. In multicomponent distillation the Fenske equation can be used to:
Estimate the fractional recoveries of the NKs at total reflux.
Calculate the number of equilibrium contacts at minimum reflux.
Estimate the average K value of the LK at total reflux.
All of the above.
None of the above.
A5. With the ready availability of process simulators, why do chemical engineers still use the Fenske-Underwood-Gilliland (FUG) method?
A6. Suppose you are doing a ternary distillation where component B, the LK, has a 98.3% recovery in the distillate, and component C, the HK, has a 99.8% recovery in the bottoms. If αA−ref = αB−ref, how does component A distribute?
A7. In Davis’s fit for the Gilliland correlation, what are the values of N and L/D when X → 0 and Y → 1? What are the values of N and L/D when X → 1 and Y → 0?
A8. An engineer claims that fit A of the Gilliland correlation is better than fit B because when they compared the predictions of both fits to detailed simulator results for a separation of interest, fit A was closer than fit B. Have they proved that fit A is better? Explain your answer.
C. Derivations
C1. Derive a form of Eq. (7-13) for (FRNK,bot) in terms of (FRLK,dist).
C2. Explore the sensitivity of Eq. (7-35) in Example 7-3 at X = 0.455 by determining Y and N as the value of the constant 0.99357 changes. Try constant values of 0.990, 0.993, and 0.994.
C3. If the pinch point occurs at the feed point, mass balances can be used to find the minimum flows. Derive these equations. Note: A pinch point at the feed can occur but is unusual in multicomponent distillation.
C4. The choice of developing the Underwood equations in terms of Vmin instead of solving for Lmin is arbitrary. Rederive the Underwood equations solving for Lmin and L—min. Develop the equations analogous to Eqs. (7-25a) and (7-28).
C5. For binary systems, Eq. (7-28) simplifies to a linear equation for both saturated liquid and saturated vapor feeds. Prove this statement.
C6. If NKs do not distribute, you solve the Underwood Eq. (7-28) for φ, which satisfies αLK−ref > φ > αHK−ref. However, if a different reference component is chosen for calculation of the relative volatilities, the value of φ changes. Despite the change in φ, Vmin calculated from Eq. (7-25a) is unchanged. The proof that this is true is challenging for the general case but is tractable for a binary system with a saturated liquid feed because Eq. (7-28) becomes linear. Prove for a binary system with a saturated liquid feed that the solution for Vmin is not affected by the choice of reference component for relative volatilities.
D. Calculation Problems
*Answers to problems with an asterisk are at the back of the book.
D1. A distillation column separates 100.0 kmol/day of a saturated liquid feed that is 20.0 mol% ethanol (E), 35.0 mol% n-propanol (P), and 45.0 mol% n-butanol (But). Fractional recovery of butanol in bottoms = 0.972. Bottoms mole fraction butanol xB,But = 0.986. Assume relative volatilities are constant: αE−But = 4.883, αP−But = 2.336, and αBut−But = 1.0.
Determine the flow rates of bottoms, B, and of distillate, D, in kmol/day; and determine the mole fractions of E, P, and But in the bottoms and in the distillate.
Find the minimum number of stages, Nmin, required for this separation.
List any assumption(s) you have made and justify why they are reasonable. Note that the strongest justification is a calculation, not just words.
D2. We are separating a mixture of propylene, propane, and isobutane in a distillation column with a partial condenser and a partial reboiler at a pressure of 15.0 bar. We desire a 0.999 fractional recovery of propylene in the distillate, at least a 0.950 fractional recovery of propane in the bottoms, and at least a 0.9999 fractional recovery of isobutane in the bottoms. How many stages are required at total reflux?
Data: At 34°C, K Propylene = 1.00, K propane = 0.89, K isobutane = 0.42.
At 40°C, K propylene = 1.13, K propane = 1.000, and K isobutane = 0.46.
D3.* A special column acts as exactly three equilibrium stages. Operating at total reflux, we measure vapor composition leaving the top stage and the liquid composition leaving the bottom stage. The column is separating phenol from o-cresol. We measure a phenol liquid mole fraction leaving the bottom stage of 0.36 and a phenol vapor mole fraction leaving the top stage of 0.545. What is the relative volatility of phenol with respect to o-cresol?
D4. Separate 1,2-dichloroethane from 1,1,2-trichloroethane at 1 atm. Distillate is 99.15 mol% 1,2-dichloroethane, and bottoms is 1.773 mol% 1,2-dichloroethane. Saturated liquid feed is 60.0 mol% 1,2-dichloroethane. Relative volatility is approximately constant, α = 2.4.
Find the minimum number of stages using the Fenske equation.
Calculate L/Dmin.
Estimate the actual number of stages for L/D = 2.2286 using the Gilliland correlation.
A detailed simulation gave 99.15 mol% 1,2-dichloroethane in the distillate, 1.773 mol% 1,2-dichloroethane in the bottoms for L/D = 2.2286, N = 25 equilibrium contacts, and optimum feed location is 16 equilibrium contacts from the top of the column. Compare this N with part c, and calculate the percent error in the Gilliland prediction.
D5. A distillation column will separate 100.0 kmol/h of a saturated liquid feed at 200 kPa that is 20.0 mol% propane (Pro), 35.0 mol% n-pentane (Pen), and 45.0 mol% n-hexane (Hex). The column has a total condenser and a partial reboiler. We want a fractional recovery of Hex in the bottoms = 0.983 and a fractional recovery of Pen in the distillate of 0.967.
Make an appropriate assumption, and determine the flow rates of bottoms, B, and of distillate, D, in kmol/h; and determine the mole fractions of bottoms and of distillate.
Determine the bubble-point temperature of the feed, and calculate relative volatilities at this temperature. Use Pen as your reference component. Report the bubble-point temperature, the K values, and the values of relative volatilities. Use a DePriester chart or Eq. (2-28). Show your work.
Assume the relative volatilities found in part b are constant, and determine the minimum number of stages, Nmin, required for this separation.
Do a calculation that justifies why the assumption made in part a is reasonable.
D6. A mixture of acetone and ethanol is distilled at 1.0 atm in a distillation column with a total condenser and a partial reboiler. We desire a distillate that is 0.999 mole fraction acetone and a bottoms that is 0.0013 mole fraction acetone. Feed is 40 mol % acetone, it is a two-phase mixture that is 5/6 liquid, and feed flow rate is 50 mol/h. Data are in Problem 4.D7.
Determine the relative volatility near the top of the column, near the bottom of the column, and near the intersection of the feed line and the equilibrium curve. Calculate the appropriate average relative volatility.
Use the Fenske equation to determine the number of equilibrium contacts at total reflux.
Assume CMO is valid and calculate the value of (L/D)min from the McCabe-Thiele diagram.
Use the Gilliland correlation (or the Davis equation) to estimate the number of stages if L/D = 1.05 (L/D)min.
Estimate the optimum feed stage location.
D7. Your boss wants some idea of how expensive it will be to distill 155.0 kmol/h of a saturated liquid feed that is 5.0 mol% methane, 10.0 mol% ethane, 15.0 mol% n-butane, 22.0 mol% n-pentane, 22.0 mol% n-hexane, and 26.0 mol% n-heptane. Column pressure is 700.0 kPa. The column has a partial condenser and a partial reboiler. We want to recover 99.0% of the n-butane in the distillate and 98.3% of the n-pentane in the bottoms. Do the calculations of the K values either from the DePriester chart or from Eq. (2-28).
Assuming that NKs do not distribute, calculate the values of D and B in kmol/h and the mole fractions in distillate and bottoms.
Do a bubble-point calculation at the feed conditions. Calculate the relative volatilities of all components with respect to the HK (n-pentane). Use these values as the average value of relative volatility for the entire column. Also determine the bubble-point temperature of the distillate to see if condensation will be expensive.
Determine the minimum number of stages for this separation with the Fenske equation.
Determine the minimum reflux ratio, (L/D)min, with the Underwood method.
Estimate the number of stages required if L/D = M × (L/D)min with the Gilliland correlation (Davis’s fit is convenient) where M = 1.04, 1.10, and 2.0.
Will this distillation be reasonably economical, or should an alternative be found? Briefly explain your reasoning.
Note: Parts b and d are easier to do with a spreadsheet or Wolfram.
D8.* We wish to separate a mixture of 40.0 mol% benzene and 60.0 mol% ethylene dichloride in a distillation column with a partial reboiler and a total condenser. The feed rate is 750 mol/h, and the feed is a saturated vapor. We desire a distillate product of 99.2 mol% benzene and a bottoms product that is 0.5 mol% benzene. Reflux is a saturated liquid, and CMO can be used. Equilibrium data can be approximated with an average relative volatility of 1.11 (benzene is more volatile).
Find the minimum external reflux ratio.
Use the Fenske equation to find the number of stages required at total reflux.
Estimate the total number of stages required for this separation using the Gilliland correlation for L/D = 1.2(L/D)min.
D9. We are separating a mixture of benzene, toluene, and xylene in a distillation rectifying column. The column has a total condenser and no reboiler. The feed is a saturated vapor that is fed into the bottom stage of the column, flow rate F = 150 kmol/h, and feed is 52.0 mol % benzene, 38.5 mol % toluene, and remainder xylenes. Pressure is 1.0 atm, CMO is valid, and the relative volatilities are constant: αBen-Tol = 2.22, αTol-Xy = 2.01. The column is at 1.0 atm. The reflux ratio L/D = 9, and the distillate is 0.007 mole fraction toluene.
Based on the best assumption you can make, use mass balances and CMO to calculate: B, mole fractions in bottoms, D, and mole fractions in distillate.
Although the column has a feed and bottoms removal, we can still operate at total reflux (D = 0 so that L/V = 1). At total reflux, how many stages are required to obtain the separation achieved in part a?
Use the Fenske equation to estimate xylene mole fraction in the distillate.
What is the minimum reflux ratio for separation in part a, but with xylene distillate mole fraction from part c?
Use the Gilliland correlation to estimate the actual number of stages if L/D = 9.
D10. When is a non-key distributing, and when is it nondistributing? For almost all chemicals, five 9s purity {concentrations of impurity below 10.0 ppm, mass (mass fraction < 1.0 × 10−5) or [approximately mole fraction < (1.0 × 10−5)]} would be low enough that the chemical can be accepted in the product and could be considered to be nondistributing (this is a very tight definition of nondistributing). A less strict concentration limit (four 9s purity) would use 100.0 ppm. Another possible definition of nondistributing that is less strict is a concentration that causes less than a 0.1% or 0.01% change in calculations of other variables (e.g., concentrations of other components, flow rates, equilibrium behavior, and so forth). Return to Example 7-1 and use the Fenske equation to explore under what conditions the NK benzene can be considered nondistributing.
Redo the calculations in Example 7-1 by assuming benzene is nondistributing, and determine new flow rates of D and B and new mole fractions in distillate and bottoms. Are the percentage changes in flow rates of B and D and percentage change in mole fractions of toluene and cumene small enough that benzene could be considered nondistributing by either the 0.1% or the 0.01% criteria?
Redo the calculations in Example 7-1 with FRtol,dist = FRcum,bot. Find the value of FRtol,dist = FRcum,bot and the corresponding value of Nmin at which benzene first meets the 10.0 ppm criterion for nondistributing.
Redo the calculations in Example 7-1 with FRtol,dist = FRcum,bot. Find the value of FRtol,dist = FRcum,bot and the corresponding value of Nmin at which benzene first meets the change of less than 0.01% in toluene and cumene distillate and bottoms concentrations criterion for nondistributing.
Find the value of FRtol,dist = FRcum,bot and the corresponding value of Nmin at which benzene first meets the change of less than 0.1% in toluene and cumene distillate and bottoms concentrations criterion for nondistributing.
D11. We simulate a distillation column and find we can obtain the desired separation with 31 stages plus a partial reboiler and a total condenser if we use an L/D = 3. With total reflux, we find that the desired separation is obtained with 13 stages plus a partial reboiler and a total condenser. Estimate (L/D)min.
D12. A distillation column is separating toluene and xylene, α = 3.03. The feed is a saturated liquid, and reflux is returned as a saturated liquid. p = 1.0 atm. F = 100.0 kmol/h. Distillate mole fraction is xD = 0.996, and bottoms xB = 0.008. Use the Underwood equation to find (L/D)min and Vmin at feed mole fractions of z = 0.1, 0.3, 0.5, 0.7, and 0.9. Check your result at z = 0.5 with a McCabe-Thiele diagram. What are the trends for |Qc,min| and QR,min as the toluene feed concentration increases? Hint: If you write the Underwood equation and solve algebraically for φ, the problem is easier than it looks.
D13.* We have a column separating benzene, toluene, and cumene. The column has a total condenser, a total reboiler, and nine equilibrium stages. The feed is 25.0 mol% benzene, 30.0 mol% toluene, and 45.0 mol% cumene. Feed rate is 100 mol/h, and the feed is a saturated liquid at 1.0 atm. The column pressure is 1.0 atm. The equilibrium data can be represented as constant relative volatilities: αBT = 2.5, αTT = 1.0, and αCT = 0.21. We desire 99.0% recovery of toluene in the distillate and 98.0% recovery of cumene in the bottoms.
Determine the required external reflux ratio.
If αBT = 2.25 instead of 2.5, what is the value of L/D?
D14. A distillation column is separating 100.0 kmol/h of a saturated vapor feed that is 30.0 mol% ethanol, 25.0 mol% i-propanol, 35.0 mol% n-propanol, and 10.0 mol% n-butanol at a pressure of 1.0 atm. We want a 98.6% recovery of i-propanol in the distillate and 99.2% recovery of n-propanol in the bottoms. The column has a total condenser and a partial reboiler. For parts b, c, and d, use the FUG method. If we choose n-propanol as the reference, the relative volatilities are ethanol = 2.17, i-propanol = 1.86, n-propanol = 1.0, and n-butanol = 0.412. These relative volatilities can be assumed to be constant.
Find D, B, xD,i, and xB,i.
Find Nmin and NF,min.
Find (L/D)min. A spreadsheet is highly recommended to find ϕ.
If L/D = 1.10(L/D)min, find N and the feed stage.
D15.* A distillation column is separating benzene (α = 2.25), toluene (α = 1.00), and cumene (α = 0.21). The column is operating at 101.3 kPa. The column has a total condenser and a partial reboiler, and the optimum feed stage is used. Reflux is a saturated liquid, and L0/D = 1.2. Feed rate is 1000.0 kmol/h. The saturated liquid feed is 39.7 mol% benzene, 16.7 mol% toluene, and 43.6 mol% cumene. Recover 99.92% of the benzene in the distillate and 99.99% of the cumene in the bottoms. For a first guess to this design problem, use the FUG approach to estimate the optimum feed stage and the total number of equilibrium stages. Note: The Underwood equations must be treated as a Case C problem.
D16.* We are separating a mixture of ethanol and n-propanol. Ethanol is more volatile, and the relative volatility is approximately constant at 2.10. The feed flow rate is 1000.0 kmol/h. The feed is 60 mol% ethanol and is a saturated vapor. We desire xD = 0.99 mole fraction ethanol, and xB = 0.008 mole fraction ethanol. The reflux is a saturated liquid. There are 30 stages in the column (including the partial reboiler). Use the FUG approach to determine:
The number of stages (including partial reboiler) at total reflux.
(L/D)min.
(L/D)actual.
D17. A distillation column operating at 200 kPa separates 100 kmol/h of a saturated liquid feed at 200 kPa that is 20 mol% propane (Pro), 35 mol% n-pentane (Pen), and 45 mol% n-hexane (Hex). The column has a total condenser and a partial reboiler. We want a fractional recovery of Hex in the bottoms = 0.983, and a fractional recovery of Pen in the distillate of 0.967.
Make an appropriate assumption and determine flow rates of bottoms, B, and of distillate, D, in kmol/h; and determine mole fractions of bottoms and of distillate.
Determine bubble-point temperature of feed and calculate relative volatilities at this temperature. Use Pen as your reference component. Report bubble-point temperature, K values, and values of relative volatilities. Use DePriester charts or Eq. (2-28). Show your work.
Assume relative volatilities found in part b are constant, and determine minimum number of stages, Nmin, required for this separation.
Do a calculation that shows assumption made in part a is correct.
D18. A depropanizer has the following feed and constant relative volatilities:
Methane (M): zM = 0.229, αM−P = 9.92
Propane (P): zP = 0.368, αP−P = 1.00
n-Butane (B): zB = 0.322, αB−P = 0.49
n-Hexane (H): zH = 0.081, αH−P = 0.10
Reflux is a saturated liquid. The feed is a saturated liquid fed at 1.0 kmol/(unit time). Assume CMO.
* L/D = 1.5, FRP,dist = 0.9854, FRB,bot = 0.8791. Use the FUG method to estimate N.
If N = 20, FRP,dist = 0.9854, and FRB,bot = 0.8791, estimate the required L/D.
Find the split of normal hexane at total reflux using Nmin.
L/D = 1.5, FRP,dist = 0.999, FRB,bot = 0.8791. Use the FUG method to estimate N.
Note: Do part a first. Parts of the solution of part a can be reused for the other parts b to d.
D19. A distillation column with a partial reboiler and a total condenser operating at 7.0 bar is separating 100.0 kmol/h of a saturated liquid feed that is 25.0 mol% ethane (C2), 35.0 mol% n-butane (C4), and 40.0 mol% n-pentane (C5). CMO can be assumed valid, and assume that ethane does not distribute. We want 99.2% recovery of n-butane in the distillate and 98.3% recovery of n-pentane in the bottoms. The K values at the distillate are KC2 = 5.56, KC4 = 0.655, and KC5 = 0.234. The K values at the bottoms are KC2 = 10.67, KC4 = 2.21, and KC5 = 0.993. Use the correct average for relative volatilities based on the values of the relative volatilities calculated at distillate and bottoms.
Find the distillate mole fractions and the value of the distillate flow rate.
Find Nmin.
Find (L/D)min.
Find N from the Gilliland correlation for M = 1.2.
E. More Complex Problems
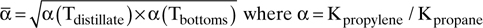
Saturated liquid feed, 50 mol% propylene, column pressure is 22.0 bar.
Saturated liquid feed, 50 mol% propylene, column pressure is 7.0 bar.
Saturated liquid feed, 50 mol% propylene, column pressure is 1.013 bar.
Saturated vapor feed, 50 mol% propylene, column pressure is 7.0 bar.
Note: This problem can be solved by brute force, or it can be simplified first and then be easily solved.
F. Problems Requiring Other Resources
F1. What variables does the Gilliland correlation not include? How might some of these be included? Check the Erbar-Maddox (1961) method (see King, 1980, or Coker, 2010).
G. Computer Simulation Problems
G1. Repeat Problem 7.D12 on Aspen Plus using RadFrac and the Peng-Robinson correlation.
Find N at total reflux (operate with very small feed and distillate rates and a large L/D).
Find (L/D)min accurately by simulating the process with a few hundred stages.
Find the actual number of stages and the optimum feed stage at L/D = 1.25(L/D)min.
G2. Repeat Problem 7. G1 except using DSTWU in Aspen Plus (Lab 6) instead of RadFrac.
