- 1.1 The Rate of Reaction, -rA
- 1.2 The General Mole Balance Equation (GMBE)
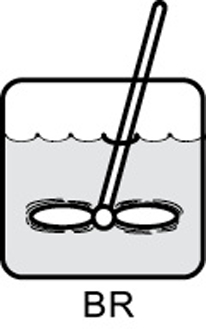
- 1.3 Batch Reactors (BRs)
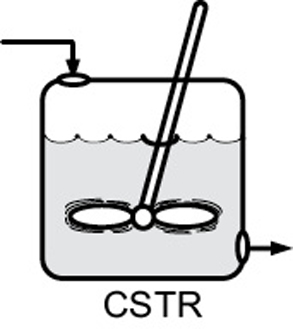
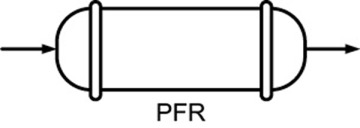
- 1.4 Continuous-Flow Reactors
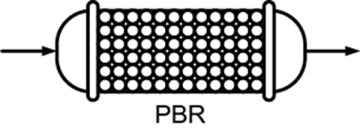
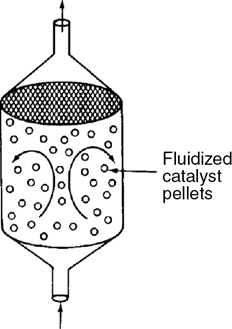
- 1.5 Industrial Reactors
- 1.6 And Now... A Word from Our Sponsor-Safety 1 (AWFOS-S1 Safety)
- Summary
- CRE Web Site Materials
- Questions, Simulations, and Problems
- Supplementary Reading
Summary
A mole balance on species j, which enters, leaves, reacts, and accumulates in a system volume V, is

If, and only if, the contents of the reactor are well mixed will the mole balance (Equation (S1-1)) on species A give

The kinetic rate law for rj is
The rate of formation of species j per unit volume (e.g., mol/s·dm3)
Solely a function of the properties of reacting materials and reaction conditions (e.g., concentration [activities], temperature, pressure, catalyst, or solvent [if any]) and does not depend on reactor type
An intensive quantity (i.e., it does not depend on the total amount)
An algebraic equation, not a differential equation (e.g., −rA = kCA or
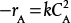 )
)
For homogeneous catalytic systems, typical units of –rj may be gram moles per second per liter; for heterogeneous systems, typical units of
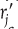 may be gram moles per second per gram of catalyst. By convention, –rA is the rate of disappearance of species A and rA is the rate of formation of species A.
may be gram moles per second per gram of catalyst. By convention, –rA is the rate of disappearance of species A and rA is the rate of formation of species A.Mole balances on species A in four common reactors are shown in Table S1-1.
TABLE S1-1 SUMMARY OF REACTOR MOLE BALANCES
|
Reactor |
Comment |
Mole Balance Differential Form |
Algebraic Form |
Integral Form |
|
BR |
No spatial variations |
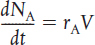
|
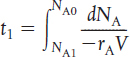
|
|
|
CSTR |
No spatial variations, steady state |
— |
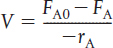
|
— |
|
PFR |
Steady state |
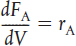
|
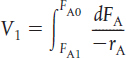
|
|
|
PBR |
Steady state |
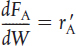
|
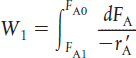
|
|
|
Fluidized CSTR |
Steady state |
— |
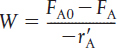
|
— |