- 1.1 The Rate of Reaction, -rA
- 1.2 The General Mole Balance Equation (GMBE)
- 1.3 Batch Reactors (BRs)
- 1.4 Continuous-Flow Reactors
- 1.5 Industrial Reactors
- 1.6 And Now... A Word from Our Sponsor-Safety 1 (AWFOS-S1 Safety)
- Summary
- CRE Web Site Materials
- Questions, Simulations, and Problems
- Supplementary Reading
1.4 Continuous-Flow Reactors
Continuous-flow reactors are almost always operated at steady state. We will consider three types: the continuous-stirred tank reactor (CSTR), the plug-flow reactor (PFR), and the packed-bed reactor (PBR). Detailed physical descriptions of these reactors can be found in both the Professional Reference Shelf (PRS), (http://www.umich.edu/~elements/6e/01chap/prof.html) for Chapter 1 and in the Visual Encyclopedia of Equipment, http://encyclopedia.che.engin.umich.edu/Pages/Reactors/CSTR/CSTR.html, and on the CRE Web site.
1.4.1 Continuous-Stirred Tank Reactor (CSTR)
A type of reactor commonly used in industrial processing is the stirred tank operated continuously (Figure 1-7). It is referred to as the continuous-stirred tank reactor (CSTR) or vat, or backmix reactor, and is primarily used for
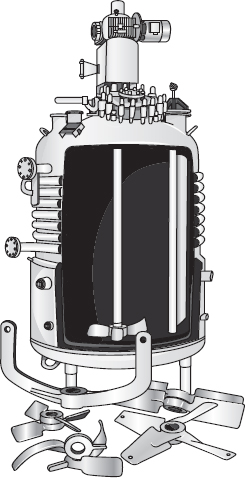
Figure 1-7(a) CSTR/batch reactor.
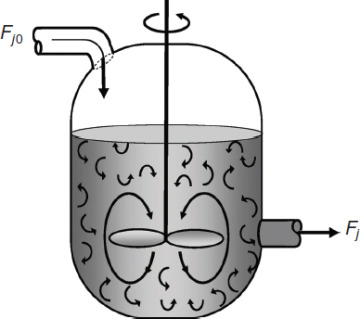
Figure 1-7(b) CSTR mixing patterns.
Also see http://encyclopedia.che.engin.umich.edu/Pages/Reactors/CSTR/CSTR.html.
liquid-phase reactions. It is normally operated at steady state and is assumed to be perfectly mixed; consequently, there is no time dependence or position dependence of the temperature, concentration, or reaction rate inside the CSTR. That is, every variable is the same at every point inside the reactor. Because the temperature and concentration are identical everywhere within the reaction vessel, they are the same at the exit point as they are elsewhere in the tank. Thus, the temperature and concentration in the exit stream are modeled as being the same as those inside the reactor. In systems where mixing is highly nonideal, the well-mixed model is inadequate, and we must resort to other modeling techniques, such as residence time distributions, to obtain meaningful results. This topic of nonideal mixing is discussed in Chapters 16 and 17, while nonideal flow reactors are discussed in Chapter 18.
When the general mole balance equation
is applied to a CSTR operated at steady state (i.e., conditions do not change with time),
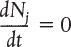
in which there are no spatial variations in the rate of reaction (i.e., perfect mixing),
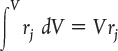
it takes the familiar form known sometimes called the design equation for a CSTR
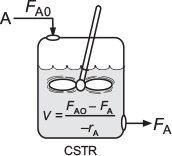
The CSTR design equation gives the reactor volume V necessary to reduce the entering molar flow rate of species j from Fj0 to the exit molar flow rate Fj, when species j is disappearing at a rate of –rj. We note that the CSTR is modeled such that the conditions in the exit stream (e.g., concentration and temperature) are identical to those in the tank. The molar flow rate Fj is just the product of the concentration of species j and the volumetric flow rate υ
Applying Equation (1-8) at the entrance of the reactor one obtains
Fj0 = Cj0 · υ0
Consequently, we can substitute for Fj0 and Fj into Equation (1-7) to write a balance on species A in terms of concentration, as
The ideal CSTR mole balance equation is an algebraic equation, not a differential equation.
1.4.2 Tubular Reactor
In addition to the CSTR and batch reactors, another type of reactor commonly used in industry is the tubular reactor. It consists of a cylindrical pipe and is normally operated at steady state, as is the CSTR. Tubular reactors are used most often for gas-phase reactions. A schematic and a photograph of industrial tubular reactors are shown in Figure 1-8.
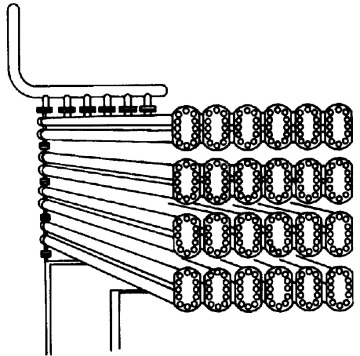
Figure 1-8(a) Tubular reactor schematic. Longitudinal tubular reactor. [Excerpted by special permission from Chem. Eng., 63(10), 211 (Oct. 1956). Copyright 1956 by McGraw-Hill, Inc., New York, NY 10020.]
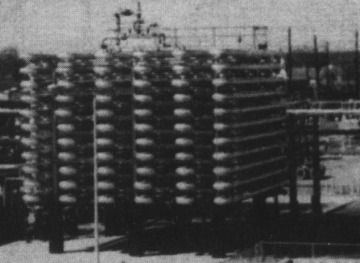
Figure 1-8(b) Tubular reactor photo. Tubular reactor for production of Dimersol G. (Photo courtesy of Editions Techniq Institut Français du Pétrole.)
Also see http://encyclopedia.che.engin.umich.edu/Pages/Reactors/PFR/PFR.html.
In the tubular reactor, the reactants are continually consumed as they flow down the length of the reactor. In modeling the tubular reactor, we assume that the concentration varies continuously in the axial direction through the reactor. Consequently, the reaction rate, which is a function of concentration for all but zero-order reactions (cf. Equation 3-2), will also vary axially. For the purposes of the material presented here, we consider systems in which the flow field may be modeled by that of a plug-flow profile (e.g., uniform radial velocity as in turbulent flow), as shown in Figure 1-9. That is, there is no radial variation in reaction rate, and the reactor is referred to as a plug-flow reactor (PFR). (The laminar-flow reactor (LFR) is discussed in Chapters 16–18, along with a discussion of nonideal reactors.)
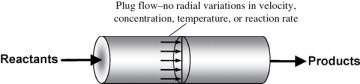
Figure 1-9 Plug-flow tubular reactor.
The general mole balance equation is given by Equation (1-4):
The equation we will use to design PFRs at steady state can be developed in two ways: (1) directly from Equation (1-4) by differentiating with respect to volume V, and then rearranging the result or (2) from a mole balance on species j in a differential segment of the reactor volume ΔV. Let’s choose the second way to arrive at the differential form of the PFR mole balance. The differential volume, ΔV, shown in Figure 1-10, will be chosen sufficiently small such that there are no spatial variations in reaction rate within this volume. Thus the generation term, ΔGj, is
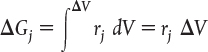
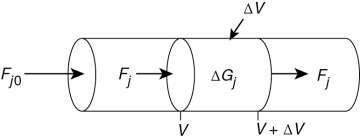
Figure 1-10 Mole balance on species j in volume ΔV.
Dividing Equation (1-10) by ΔV and rearranging
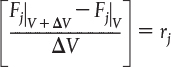
we note the term in brackets resembles the definition of a derivative
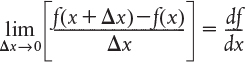
Taking the limit as ΔV approaches zero, we obtain the differential form of steady-state mole balance on a PFR

We could have made the cylindrical reactor on which we carried out our mole balance an irregularly shaped reactor, such as the one shown in Figure 1-11 for reactant species A. However, we see that by applying Equation (1-10), the result would yield the same equation (i.e., Equation (1-11)). For species A, the mole balance is
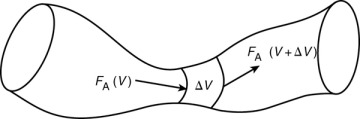
Figure 1-11 Pablo Picasso’s reactor.
Consequently, we see that Equation (1-11) applies equally well to our model of tubular reactors of variable and constant cross-sectional area, although it is doubtful that one would find a reactor of the shape shown in Figure 1-11 unless it were designed by Pablo Picasso or perhaps one of his followers.
The conclusion drawn from the application of the design equation to Picasso’s reactor is an important one: the degree of completion of a reaction achieved in an ideal plug-flow reactor (PFR) does not depend on its shape, only on its total volume.
Lets again consider the isomerization A → B, this time in a PFR. As the reactants proceed down the reactor, A is consumed by chemical reaction and B is produced. Consequently, the molar flow rate FA decreases as shown in Figure 1-12(a) while FB increases as the reactor volume V increases, as shown in Figure 1-12(b).
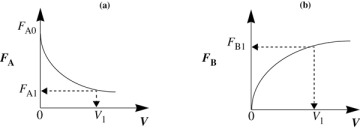
Figure 1-12 Profiles of molar flow rates in a PFR.
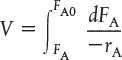
We now ask, “What is the reactor volume V1 necessary to reduce the entering molar flow rate of A from FA0 to an exit flow rate FA1?” Rearranging Equation (1-12) in the form
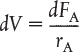
and integrating with limits at V = 0, then FA = FA0, and at V = V1, then FA = FA1
V1 is the volume necessary to reduce the entering molar flow rate FA0 to some specified value FA1 and also the volume necessary to produce a molar flow rate of B of FB1.
1.4.3 Packed-Bed Reactor (PBR)
The principal difference between reactor design calculations involving homogeneous reactions and those involving fluid–solid heterogeneous reactions is that for the latter, the reaction takes place on the surface of the catalyst (see Figure 10-5). The greater the mass of a given catalyst, the greater the reactive surface area. Consequently, the reaction rate is based on mass of solid catalyst, W, rather than on reactor volume, V. For a fluid–solid heterogeneous system, the rate of reaction of a species A, 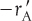 , is defined as
, is defined as
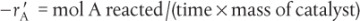
The mass of solid catalyst is used because the amount of catalyst is what is important to the rate of product formation. We note that by multiplying the heterogeneous reaction rate, 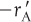 , by the bulk catalyst density,
, by the bulk catalyst density, 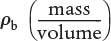 , we can obtain the reaction rate per unit volume, –rA.
, we can obtain the reaction rate per unit volume, –rA.
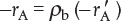
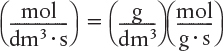
The reactor volume that contains the catalyst is of secondary significance. Figure 1-13 shows a schematic of an industrial catalytic reactor with vertical tubes packed with solid catalyst.
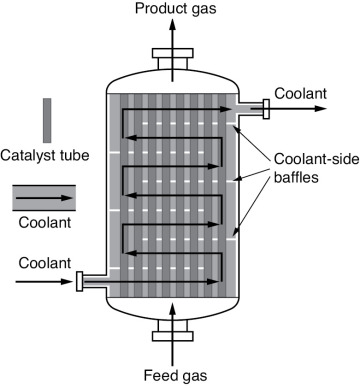
Figure 1-13 Longitudinal catalytic packed-bed reactor. Also see http://encyclopedia.che.engin.umich.edu/Pages/Reactors/PBR/PBR.html.
In the three idealized types of reactors just discussed (the perfectly mixed batch reactor [BR], the plug-flow tubular reactor [PFR]), and the perfectly mixed continuous-stirred tank reactor [CSTR]), the design equations (i.e., mole balances) were developed based on reactor volume. The derivation of the design equation for a packed-bed catalytic reactor (PBR) will be carried out in a manner analogous to the development of the tubular design equation. To accomplish this derivation, we simply replace the volume coordinate, V, in Equation (1-10) with the catalyst mass (i.e., weight) coordinate W (Figure 1-14).
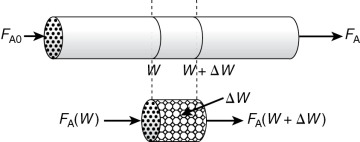
Figure 1-14 Packed-bed reactor schematic.
As with the PFR, the PBR is assumed to have no radial gradients in concentration, temperature, or reaction rate. The generalized mole balance on species A over catalyst weight ΔW results in the equation
The dimensions of the generation term in Equation (1-14) are
which are, as expected, the same dimensions of the molar flow rate FA. After dividing Equation (1-14) by ΔW and taking the limit as ΔW → 0, we arrive at the differential form of the mole balance for a packed-bed reactor:
When pressure drop through the reactor (see Section 5.5) and catalyst decay (see Section 10.7 in Chapter 10) are neglected, the integral form of the packed-catalyst-bed design equation can be used to calculate the catalyst weight
W is the catalyst weight necessary to reduce the entering molar flow rate of species A, FA0, down to a molar flow rate FA.
1.4.4 Well-Mixed “Fluidized” Catalytic Bed Reactor
For particulate catalytic gas-phase systems, the fluidized bed is also in common use. Depending of the flow regime, it can be modeled anywhere between a straight through transport reactor (Chapter 10) to a fluidized bed that is analogous to a CSTR (section 1.4.1), which is shown in Figure 1-15.
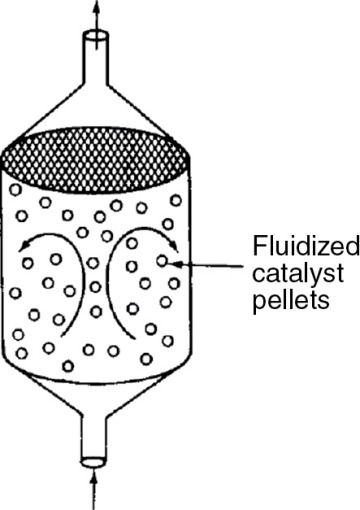
Figure 1-15 Well-mixed fluidized bed modeled as a CSTR.
A mole balance on species A in a well-mixed “fluidized” bed is
Dividing by the catalyst weight W, we arrive at the Equation (1-18) that gives the catalyst weight necessary to reduce the molar rate entering from, FA0 (mol/s) to the molar rate leaving, FA, (mol/s) when species A is disappearing at a rate, 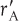 (mol/s·gcat) the design equation
(mol/s·gcat) the design equation
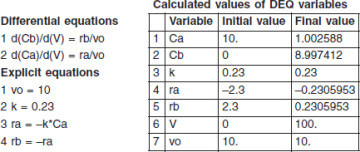
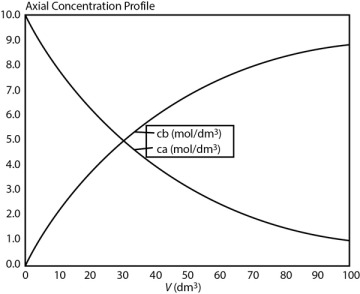
For some insight into “things to come,” consider the following example of how one can use the tubular reactor design in Equation (1-11).

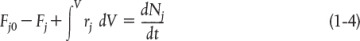



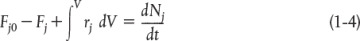



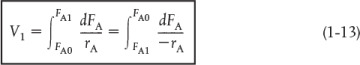
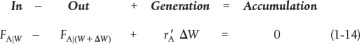
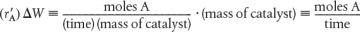

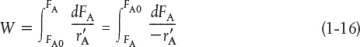


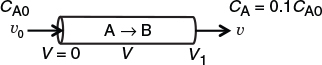
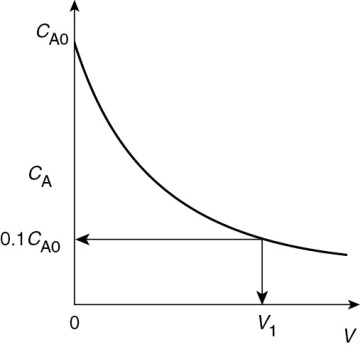


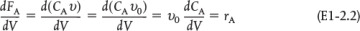

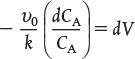


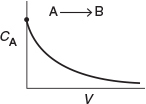
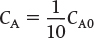 when k = 0.23 min–1 and υ0 = 10 dm3/min.
when k = 0.23 min–1 and υ0 = 10 dm3/min.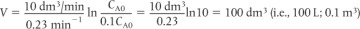 Ans. We see that a reactor volume of 0.1 m3 is necessary to convert 90% of species A entering (i.e., CA = 0.1 CA0) into product B for the parameters given.
Ans. We see that a reactor volume of 0.1 m3 is necessary to convert 90% of species A entering (i.e., CA = 0.1 CA0) into product B for the parameters given.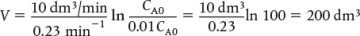 Ans.
Ans.