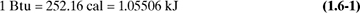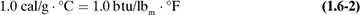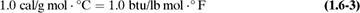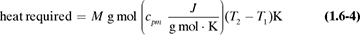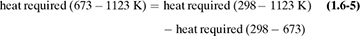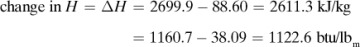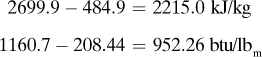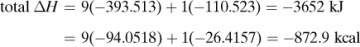- 1.0 Chapter Objectives
- 1.1 Classification of Transport Processes and Separation Processes (Unit Operations)
- 1.2 SI System of Basic Units Used in This Text and Other Systems
- 1.3 Methods of Expressing Temperatures and Compositions
- 1.4 Gas Laws and Vapor Pressure
- 1.5 Conservation of Mass and Material Balances
- 1.6 Energy and Heat Units
- 1.7 Conservation of Energy and Heat Balances
- 1.8 Numerical Methods for Integration
- 1.9 Chapter Summary
- Problems
- References
- Notation
1.6 Energy and Heat Units
1.6A Joule, Calorie, and Btu
In a manner similar to that used in making material balances on chemical and biological processes, we can also make energy balances on a process. Often, a large portion of the energy entering or leaving a system is in the form of heat. Before such energy or heat balances are made, we must understand the various types of energy and heat units.
In the SI system, energy is given in joules (J) or kilojoules (kJ). Energy is also expressed in btu (British thermal units) or cal (calories). The calorie (abbreviated as cal) is defined as the amount of heat needed to heat 1.0 g water 1.0°C (from 14.5°C to 15.5°C). Also, 1 kcal (kilocalorie) = 1000 cal. The btu is defined as the amount of heat needed to raise 1.0 lb water 1°F. Hence, from Appendix A.1,
1.6B Heat Capacity
The heat capacity of a substance is defined as the amount of heat necessary to increase the temperature by 1 degree. It can be expressed for 1 g, 1 lb, 1 g mol, 1 kg mol, or 1 lb mol of the substance. For example, a heat capacity is expressed in SI units as J/kg mol · K; in other units as cal/g · °C, cal/g mol · °C, kcal/kg mol · °C, Btu/lbm · °F, or btu/lb mol · °F.
It can be shown that the actual numerical value of a heat capacity is the same in mass units or in molar units. That is,
For example, to prove this, suppose that a substance has a heat capacity of 0.8 btu/lbm · °F. The conversion is made using 1.8°F for 1°C or 1 K, 252.16 cal for 1 btu, and 453.6 g for 1 lbm, as follows:
The heat capacities of gases (also called specific heat) at constant pressure cp are functions of temperature and, for engineering purposes, can often be assumed to be independent of pressure up to several atmospheres. In most process-engineering calculations, one is usually interested in the amount of heat needed to heat a gas from one temperature t1 to another at t2. Since the cp varies with temperature, an integration must be performed or a suitable mean cpm used. These mean values for gases have been obtained for T1 of 298 K or 25°C (77°F) and various T2 values, and are tabulated in Table 1.6-1 at 101.325 kPa pressure or less as cpm in kJ/kg mol · K at various values of T2 in K or °C.
Table 1.6-1. Mean Molar Heat Capacities of Gases Between 298 and TK (25 and T°C) at 101.325 kPa or Less (SI Units: cp = kJ/kg mol · K)
T(K) |
T(°C) |
H2 |
N2 |
CO |
Air |
O2 |
H2O |
CO2 |
CH4 |
SO2 |
|---|---|---|---|---|---|---|---|---|---|---|
298 |
25 |
28.86 |
29.14 |
29.16 |
29.19 |
29.38 |
33.59 |
37.20 |
35.8 |
39.9 |
373 |
100 |
28.99 |
29.19 |
29.24 |
29.29 |
29.66 |
33.85 |
38.73 |
37.6 |
41.2 |
473 |
200 |
29.13 |
29.29 |
29.38 |
29.40 |
30.07 |
34.24 |
40.62 |
40.3 |
42.9 |
573 |
300 |
29.18 |
29.46 |
29.60 |
29.61 |
30.53 |
34.39 |
42.32 |
43.1 |
44.5 |
673 |
400 |
29.23 |
29.68 |
29.88 |
29.94 |
31.01 |
35.21 |
43.80 |
45.9 |
45.8 |
773 |
500 |
29.29 |
29.97 |
30.19 |
30.25 |
31.46 |
35.75 |
45.12 |
48.8 |
47.0 |
873 |
600 |
29.35 |
30.27 |
30.52 |
30.56 |
31.89 |
36.33 |
46.28 |
51.4 |
47.9 |
973 |
700 |
29.44 |
30.56 |
30.84 |
30.87 |
32.26 |
36.91 |
47.32 |
54.0 |
48.8 |
1073 |
800 |
29.56 |
30.85 |
31.16 |
31.18 |
32.62 |
37.53 |
48.27 |
56.4 |
49.6 |
1173 |
900 |
29.63 |
31.16 |
31.49 |
31.48 |
32.97 |
38.14 |
49.15 |
58.8 |
50.3 |
1273 |
1000 |
29.84 |
31.43 |
31.77 |
31.79 |
33.25 |
38.71 |
49.91 |
61.0 |
50.9 |
1473 |
1200 |
30.18 |
31.97 |
32.30 |
32.32 |
33.78 |
39.88 |
51.29 |
64.9 |
51.9 |
1673 |
1400 |
30.51 |
32.40 |
32.73 |
32.76 |
34.19 |
40.90 |
52.34 |
|
|
Mean Molar Heat Capacities of Gases Between 25 and T°C at 1 atm Pressure or Less (English Units: cp = btu/lb mol · °F)
T(°C) |
H2 |
N2 |
CO |
Air |
O2 |
NO |
H2O |
CO2 |
HCl |
Cl2 |
CH4 |
SO2 |
C2H4 |
SO3 |
C2H6 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
25 |
6.894 |
6.961 |
6.965 |
6.972 |
7.017 |
7.134 |
8.024 |
8.884 |
6.96 |
8.12 |
8.55 |
9.54 |
10.45 |
12.11 |
12.63 |
100 |
6.924 |
6.972 |
6.983 |
6.996 |
7.083 |
7.144 |
8.084 |
9.251 |
6.97 |
8.24 |
8.98 |
9.85 |
11.35 |
12.84 |
13.76 |
200 |
6.957 |
6.996 |
7.017 |
7.021 |
7.181 |
7.224 |
8.177 |
9.701 |
6.98 |
8.37 |
9.62 |
10.25 |
12.53 |
13.74 |
15.27 |
300 |
6.970 |
7.036 |
7.070 |
7.073 |
7.293 |
7.252 |
8.215 |
10.108 |
7.00 |
8.48 |
10.29 |
10.62 |
13.65 |
14.54 |
16.72 |
400 |
6.982 |
7.089 |
7.136 |
7.152 |
7.406 |
7.301 |
8.409 |
10.462 |
7.02 |
8.55 |
10.97 |
10.94 |
14.67 |
15.22 |
18.11 |
500 |
6.995 |
7.159 |
7.210 |
7.225 |
7.515 |
7.389 |
8.539 |
10.776 |
7.06 |
8.61 |
11.65 |
11.22 |
15.60 |
15.82 |
19.39 |
600 |
7.011 |
7.229 |
7.289 |
7.299 |
7.616 |
7.470 |
8.678 |
11.053 |
7.10 |
8.66 |
12.27 |
11.45 |
16.45 |
16.33 |
20.58 |
700 |
7.032 |
7.298 |
7.365 |
7.374 |
7.706 |
7.549 |
8.816 |
11.303 |
7.15 |
8.70 |
12.90 |
11.66 |
17.22 |
16.77 |
21.68 |
800 |
7.060 |
7.369 |
7.443 |
7.447 |
7.792 |
7.630 |
8.963 |
11.53 |
7.21 |
8.73 |
13.48 |
11.84 |
17.95 |
17.17 |
22.72 |
900 |
7.076 |
7.443 |
7.521 |
7.520 |
7.874 |
7.708 |
9.109 |
11.74 |
7.27 |
8.77 |
14.04 |
12.01 |
18.63 |
17.52 |
23.69 |
1000 |
7.128 |
7.507 |
7.587 |
7.593 |
7.941 |
7.773 |
9.246 |
11.92 |
7.33 |
8.80 |
14.56 |
12.15 |
19.23 |
17.86 |
24.56 |
1100 |
7.169 |
7.574 |
7.653 |
7.660 |
8.009 |
7.839 |
9.389 |
12.10 |
7.39 |
8.82 |
15.04 |
12.28 |
19.81 |
18.17 |
25.40 |
1200 |
7.209 |
7.635 |
7.714 |
7.719 |
8.068 |
7.898 |
9.524 |
12.25 |
7.45 |
8.94 |
15.49 |
12.39 |
20.33 |
18.44 |
26.15 |
1300 |
7.252 |
7.692 |
7.772 |
7.778 |
8.123 |
7.952 |
9.66 |
12.39 |
|
|
|
|
|
|
|
1400 |
7.288 |
7.738 |
7.818 |
7.824 |
8.166 |
7.994 |
9.77 |
12.50 |
|
|
|
|
|
|
|
1500 |
7.326 |
7.786 |
7.866 |
7.873 |
8.203 |
8.039 |
9.89 |
12.69 |
|
|
|
|
|
|
|
1600 |
7.386 |
7.844 |
7.922 |
7.929 |
8.269 |
8.092 |
9.95 |
12.75 |
|
|
|
|
|
|
|
1700 |
7.421 |
7.879 |
7.958 |
7.965 |
8.305 |
8.124 |
10.13 |
12.70 |
|
|
|
|
|
|
|
1800 |
7.467 |
7.924 |
8.001 |
8.010 |
8.349 |
8.164 |
10.24 |
12.94 |
|
|
|
|
|
|
|
1900 |
7.505 |
7.957 |
8.033 |
8.043 |
8.383 |
8.192 |
10.34 |
13.01 |
|
|
|
|
|
|
|
2000 |
7.548 |
7.994 |
8.069 |
8.081 |
8.423 |
8.225 |
10.43 |
13.10 |
|
|
|
|
|
|
|
2100 |
7.588 |
8.028 |
8.101 |
8.115 |
8.460 |
8.255 |
10.52 |
13.17 |
|
|
|
|
|
|
|
2200 |
7.624 |
8.054 |
8.127 |
8.144 |
8.491 |
8.277 |
10.61 |
13.24 |
|
|
|
|
|
|
|
Source: O. A. Hougen, K. W. Watson, and R. A. Ragatz, Chemical Process Principles, Part I, 2nd ed. New York: John Wiley & Sons, Inc., 1954.
When heating a gas mixture, the total heat required is determined by first calculating the heat required for each individual component and then adding the results to obtain the total.
The heat capacities of solids and liquids are also functions of temperature and independent of pressure. Data are given in Appendix A.2, Physical Properties of Water; A.3, Physical Properties of Inorganic and Organic Compounds; and A.4, Physical Properties of Foods and Biological Materials. More data are available in (P1) in the References section at the end of this chapter.
The enthalpy, H, of a substance in J/kg represents the sum of the internal energy plus the pressure–volume term. For no reaction and a constant-pressure process with a change in temperature, the heat change as computed from Eq. (1.6-4) is the difference in enthalpy, ΔH, of the substance relative to a given temperature or base point. In other units, H = btu/lbm or cal/g.
1.6C Latent Heat and Steam Tables
Whenever a substance undergoes a change of phase, relatively large amounts of heat change are involved at a constant temperature. For example, ice at 0°C and 1 atm pressure can absorb 6013.4 kJ/kg mol. This enthalpy change is called the latent heat of fusion. Data for other compounds are available in various handbooks (P1, W1).
When a liquid phase vaporizes to a vapor phase under its vapor pressure at constant temperature, an amount of heat called the latent heat of vaporization must be added. For water at 25°C and a pressure of 23.75 mmHg, the latent heat is 44 020 kJ/kg mol, and at 25°C and 760 mmHg, 44 045 kJ/kg mol. Hence, the effect of pressure can be neglected in these types of engineering calculations. However, there is a large effect of temperature on the latent heat of water. Also, the effect of pressure on the heat capacity of liquid water is small and can be neglected.
Since water is a very common chemical, the thermodynamic properties of it have been compiled in steam tables and are given in Appendix A.2 in SI and in English units.
1.6D Heat of Reaction
When chemical reactions occur, heat effects always accompany these reactions. This area where energy changes occur is often called thermochemistry. For example, when HCl is neutralized with NaOH, heat is given off and the reaction is exothermic. Heat is absorbed in an endothermic reaction. This heat of reaction is dependent on the chemical nature of each reacting material and product, and on their physical states.
For purposes of organizing data, we define a standard heat of reaction ΔH0 as the change in enthalpy when 1 kg mol reacts under a pressure of 101.325 kPa at a temperature of 298 K (25°C). For example, for the reaction
the ΔH0 is –285.840 × 103 kJ/kg mol or –68.317 kcal/g mol. The reaction is exothermic and the value is negative since the reaction loses enthalpy. In this case, the H2 gas reacts with the O2 gas to give liquid water, all at 298 K (25°C).
Special names are given to ΔH0 depending upon the type of reaction. When the product is formed from the elements, as in Eq. (1.6-6), we call ΔH0 the heat of formation of the product water, 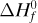 . For the combustion of CH4 to form CO2 and H2O, we call it heat of combustion,
. For the combustion of CH4 to form CO2 and H2O, we call it heat of combustion, 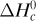 . Data are given in Appendix A.3 for various values of
. Data are given in Appendix A.3 for various values of 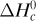 .
.
If a table of heats of formation, 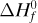 , of compounds is available, the standard heat of the reaction, ΔH0, can be calculated by
, of compounds is available, the standard heat of the reaction, ΔH0, can be calculated by
In Appendix A.3, a short table of some values of ΔHf is given. Other data are also available (H1, P1, S1).