Hunting for Disease Genes
- Venezuelan adventures: the isolation of the Huntington's gene
- Ethnicity, religion, and the gene-hunting companies
- The biggest pedigree of all: deCODE genetics and the Icelandic population
- How many disease genes are there?
Leopold George Duncan Albert, Duke of Albany, eighth child and youngest son of Queen Victoria, was buried on Saturday, April 12, 1884, in the Albert Memorial Chapel, Windsor Castle.1 He was only 31. Leopold's pregnant wife Princess Helene, the daughter of George Victor, reigning Prince of Waldeck-Pyrmont, arrived by carriage to view her husband's remains and to shed some tears over them. Next, the Seaforth Highlanders, in which Leopold was an honorary colonel, arrived. They were wearing their medals and sidearms. The Coldstream Guards followed the Seaforths led by their band. The servants of the late Prince Albert, the servants of the Queen, and then the gentlemen of Leopold's household followed them. The coffin was borne by eight Seaforth Highlanders and followed by the Prince of Wales in the uniform of a field marshal.
Also marching in the funeral procession was a French general who had accompanied Leopold's remains from Cannes, where he had died. On March 27, Leopold had slipped on a tiled floor in the Yacht Club and injured his knee. Although it has been claimed that Prince Leopold died from the effects of the morphine he had been given to ease the pain on top of the claret he had consumed with his dinner, it seems more likely that he died of a cerebral hemorrhage.2 Leopold was the first victim of what has been called the "Royal Disease" or hemophilia.
Hemophilia A and B, recessive, sex-linked diseases, are normally expressed only in males because a male has a single X chromosome, whereas a female has two, one usually having the normal gene. That is, women are carriers who do not show any symptoms of hemophilia. Hemophilia spread from the British royal line into the Russian, Prussian, and Spanish royal lines through intermarriage. Its source was Queen Victoria. She had two daughters who were carriers in addition to Leopold, but her other five offspring did not express or transmit the defective gene to their progeny.
Although it is remotely possible that a hemophilia mutation occurred in Queen Victoria very early in egg formation, it is much more likely that Queen Victoria was a carrier of the hemophilia mutation because three of her children had the hemophilia gene. If the Queen was a carrier, the egg from which she arose would either have had to be fertilized by a mutant sperm from her father Edward, Duke of Kent, or else her father was not the duke. After spending many years in Europe in the company of various mistresses, notably Adelaide Dubus and Julie St. Laurent, Edward married Victoire (or Victoria) of Saxe-Coburg-Saalfeld, the widow of the Prince of Leningen, in 1818. Victoria was born the next year and Edward died in 1820.
Perhaps the sperm that fertilized the egg that produced Queen Victoria possessed the hemophilia mutation. If so, the mutation would have arisen during spermatogenesis in the duke as there is no prior evidence of hemophilia in the royal line. In his book The Victorians, A. N. Wilson proposes a different theory. Another man may have fathered Victoria. Wilson supposes that man may have been her mother's secretary Sir John Conroy, a man Queen Victoria detested. Conroy and Victoire were widely suspected of being lovers, but there is no evidence he had hemophilia. Even if Conroy was not Queen Victoria's father, Wilson writes, "it seems overwhelmingly probable that Victoire, uncertain of her husband's potency or fertility, took a lover to determine that the Coburg dynasty would eventually take over the throne of England."3 If so, the presumptive interloper would have needed to work quickly. After all, Victoria of Saxe-Coburg-Saalfeld and Edward, Duke of Kent, were married on May 29, 1818, and Queen Victoria was born just a year later.
It has long been assumed that hemophilia A rather than hemophilia B was the disease transmitted by Queen Victoria because hemophilia A accounts for 85% of all cases and hemophilia B for about 14% with various other clotting defects accounting for the remaining 1%.4 However, we now know that Queen Victoria carried a hemophilia B mutation. This finding emerges from some remarkable detective work involving the remains of the murdered family of Nicholas II, the last Russian czar.
On July 16, 1918, the czar, his family, the royal physician, and three servants were herded into the cellar of Ipatiev House in Yekaterinburg where they were held prisoner and shot by a firing squad.5 The bodies were to be thrown down a mine shaft, but the truck that carried them began to have engine problems so the murderers dug a shallow pit as a grave, poured sulfuric acid on the bodies to impede their identification, covered the bodies, and drove the truck back and forth over the grave site to flatten it. Half a year later, a Russian investigator, Nicholas Sokolov, retrieved some valuable objects from the likely tomb, but reported no evidence of skeletal remains. He concluded that the bodies had been destroyed, but in April 1989, a filmmaker named Geli Ryabov claimed that the bodies had not been destroyed, but that they were located five miles from the site discovered by Sokolov. Ryabov and a geologist colleague had worked out the actual burial place from photographs and the original report written by the head executioner.
DNA analysis confirmed the presence of the skeletal remains of nine people. They included the czar, the czarina, three of their five children, the royal physician, and three servants. However, two of the children were missing. This was in accord with the executioner's report that he had burned two of the bodies, one of which belonged to the czar's only son Alexei, a hemophiliac. Burned bone fragments from two skeletons were found in 2007 in another grave at the site of a bonfire in the same area.6 The fragments proved to be what was left of Alexei and his sister Alexandra.
The hemophilia A and B genes are called F8 and F9 because they encode clotting factors 8 and 9, respectively. DNA analysis of the F8 and F9 genes recovered from the remains revealed that only the latter gene was altered and that Alexandra was a carrier, whereas Alexei's single X chromosome had, of course, the hemophilia mutation.7
The pedigree of the "Royal Disease" illustrates how useful a good lineage is in attributing a specific disease to a defective gene. This chapter considers two different approaches to identifying disease and susceptibility genes. The first is to target a specific gene. The example given here is the discovery of the gene whose alteration results in Huntington's chorea. The pedigree that provided the answer was found on the shores of Lake Maracaibo in Venezuela. Once an approximate chromosomal location had been established for the gene, the investigators, led by James Gusella at Harvard and Nancy Wexler at Columbia, had to inch along the chromosome to the actual gene using various molecular techniques, a method referred to as "positional cloning" (see Glossary).
The second approach is to search for a variety of deleterious genes in a specific sect or group that exhibits characteristics such as originating from a small founding group, inbreeding, or a high incidence of several different disease genes. The Amish, Ashkenazi Jews, and French Canadians are examples. This is one approach favored by many gene-hunting companies. Once again, pedigree analysis and positional cloning play key roles.
Until the last ten years or so, these were the two major approaches to gene identification, but with the discovery that the human genome is riddled with small genetic differences called single nucleotide polymorphisms or SNPs (see Glossary and Chapter 2, "How genetic diseases arise") coupled with the publication of the human genome sequence, two other approaches became popular that do not require information from pedigrees. In the first, called the candidate gene method, the investigator makes an educated guess at a gene or genes mutation of which might lead to a specific genetic disability. The gene and surrounding DNA are compared between people with and without the condition to see whether there are any alterations specific to people having the disease. The second method is completely unbiased and involves comparing entire genomes between the two groups for differences in SNPs. These genome-wide association studies (GWAS) have the potential for discovering differences related to genes that might not normally have been suspected of causing the disease. These methods, especially the latter, are particularly well adapted to finding genetic factors underlying complex genetic diseases like type 2 diabetes (see Chapter 4, "Susceptibility genes and risk factors," for a fuller discussion). However, as the price of whole genome sequencing continues to drop rapidly, whole genome sequencing comparisons will probably replace the candidate gene and GWAS approaches.
Venezuelan adventures: the isolation of the Huntington's gene
One day in 1858, George Huntington, a boy of eight, was riding with his father George Lee Huntington, a physician. His father was making his medical rounds on a wooded road between the towns of Amagansett and Easthampton on the South Fork of Long Island when "we suddenly came upon a mother and a daughter, both bowing, twisting, grimacing. I stared in wonderment, almost in fear. What could it mean?"8 Thus was George Huntington introduced to the disease that would later bear his name, Huntington's chorea. Huntington's grandfather, a physician like his father, migrated to the eastern end of Long Island from Connecticut in 1797. Both his grandfather and father had observed the "slow onset and gradual development" of this hereditary disease and how some of its victims "worked on their trades long after the choreic movements had developed, but gradually succumbed to the inevitable, becoming more and more helpless as time advanced, and often mind and body failed at an even pace."
Like his father and grandfather before him, George Huntington became a doctor after obtaining his medical degree at Columbia University in 1871. That same year, he moved to Pomeroy, Ohio, to set up a family practice. On February 15, 1872, he traveled five miles across the icy landscape to Middleport, Ohio, to deliver a paper to the Meigs and Mason Academy of Medicine. The academy's membership was made up of physicians from two sparsely populated counties of the same name. In his report titled "On Chorea," Huntington began with a general review pointing out that "chorea" was a disease of the nervous system whose name derived from "the dancing propensities of those who are affected by it." He noted that chorea was principally a disease of childhood. In contrast, "hereditary chorea" as he called it was confined to the few families he had observed in Easthampton as "an heirloom from generations away back in the dim past" and it did not manifest itself until "adult or middle life."
Huntington's presentation was well received, so he submitted the manuscript to the editors of the Medical and Surgical Reporter of Philadelphia, where it was published on April 13, 1872.9 Huntington's paper describing what he called "hereditary chorea" was short, clear, and concise and was widely discussed, abstracted for international yearbooks, and published in its entirety in various texts. In 1915, Charles Benedict Davenport, Director of the Eugenics Records Office at Cold Spring Harbor, New York, and a member of the National Academy of Sciences, published a paper on Huntington's chorea in the first volume of its Proceedings.10 Pedigree data from four families suggested strongly that a dominant gene mutation was responsible for the disease, a hypothesis that has proved to be correct.
The discovery of the defective gene that causes Huntington's chorea really begins with the folk singer and songwriter Woody Guthrie.11 In 1956, he was arrested in New Jersey for "wandering aimlessly," a charge often brought against the mentally ill or confused. He was committed to the Greystone Park Psychiatric Hospital in Morris Plains, New Jersey, a sprawling complex of 43 buildings that opened in 1876. He remained there until 1961 by which time his condition had worsened and he was transferred to the Creedmore facility on Long Island, where he died in 1967. Following Guthrie's death, his widow formed the Committee to Combat Huntington's Disease. Milton Wexler, a doctor, joined Guthrie in her quest. His wife and three brothers-in-law were suffering from the disease.12 Wexler's daughter Nancy was in graduate school when her mother was diagnosed with Huntington's disease. She realized she had a 50% chance of having the Huntington's mutation herself. In her PhD dissertation at the University of Michigan in clinical psychology, she explored the cognitive and emotional consequences of being at risk for Huntington's disease. She has never revealed publicly whether she has been tested for the gene. However, it seems unlikely that she will become a disease victim because she is now over 60 and has not expressed its symptoms.
Nancy Wexler was determined to try to identify the Huntington's gene. Her big break came in 1972 when Dr. Americo Negrette, a Venezuelan physician, presented a paper at a conference in the United States.13 Dr. Negrette had set up his practice in 1952 in a remote community near the great saltwater gulf called Lake Maracaibo. He soon noticed that certain individuals were stumbling, weaving, and falling down, and concluded they were probably drunk. He learned from the residents, however, that they were not drunk, but suffered from a disease that was locally called El Mal. He soon realized that they were expressing the symptoms of Huntington's disease and published a book on the subject in 1955.
Dr. Negrette had already begun to construct a pedigree for Huntington's disease in the Lake Maracaibo population when Nancy Wexler and her team joined him 1979. Members of the relevant families live in three villages on the shores of the lake. The scientists succeeded in tracing the pedigree back to a woman named Maria Concepcion who lived in the early 1800s and had ten children. She may not have had the disease herself. It is likely that the children of hers who suffered from the disease may have inherited it from their father, possibly a sailor from Europe. By 2004, this pedigree numbered 18,149 of whom 15,409 were still living.14
The blood samples from the pedigree were shipped to James Gusella's laboratory at Massachusetts General Hospital. In only four years, by mid-1983, Gusella had located a region near the end of the short arm of chromosome 4 that was close to the gene,15 but, given the technological limitations at the time, it took another ten years to find and sequence the gene itself.16 Some idea of the enormous amount of work that went into locating and characterizing the Huntington's gene is apparent from the authorship of the paper, which is given simply as "The Huntington's Disease Collaborative Research Group." It turns out this is the collective title for six groups located at different institutions. There are multiple named authors from each institution.
The nature of the defect in Huntington's chorea was unexpected. In the middle of the gene is the sequence CAG. The four bases in DNA are cytosine (C), guanine (G), adenine (A), and thymine (T). In the genetic code, they are read in groups of three. Each base is attached via a sugar molecule (deoxyribose) to a phosphorous group that hooks the whole structure into the DNA backbone (see Figure 1–1). This structure is referred to as a nucleotide with a group of three nucleotides being a trinucleotide. The CAG sequence specifies the amino acid glutamine in the middle of a nerve cell protein that was named huntingtin and was encoded by the Huntington's gene. The CAG sequence in the gene and the corresponding glutamine sequence in the protein are repeated a number of times. In Huntington's disease, there are more CAG repeats than normal. The longer the CAG stretch, the earlier the onset of the disease. This type of trinucleotide repeat mutation is not unique to Huntington's disease, but is characteristic of certain other genetic diseases as well (see Table 1–1).
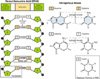
Figure 1–1 Left. A short sequence from the DNA double helix showing the four bases, adenine (A), thymine (T), guanine (G), and cytosine (C). Each base is bonded to a sugar molecule (deoxyribose) which is linked in turn to a phosphate atom to form a nucleotide. The nucleotides are linked to each other via strong, covalent bonds to form the sugar-phosphate backbone of each strand of the helix. The two strands of the helix are held together by hydrogen bonds between the bases with A pairing with T and G with C. Right. G-C and A-T base pairs in more detail. Note that hydrogen bonds are much weaker than covalent bonds and that uracil (U) replaces thymine in RNA.
Courtesy: National Human Genome Research Institute.
Table 1–1. Examples of trinucleotide repeat diseases*
|
Number of repeats |
|||
|
Disease |
Trinucleotide |
Normal |
Disease |
|
Huntington's disease |
CAG |
10–35 |
40–121 |
|
Fragile X syndrome |
CGG |
5–54 |
>200–2,000 |
|
Friedreich's ataxia |
GAA |
7–34 |
200–1,700 |
|
Myotonic dystrophy |
CTG |
5–37 |
50–11,000 |